Learn more about Phynova Joint and Muscle Pain Relief Tablets
Video for healthcare professionals
Pharmacy team training
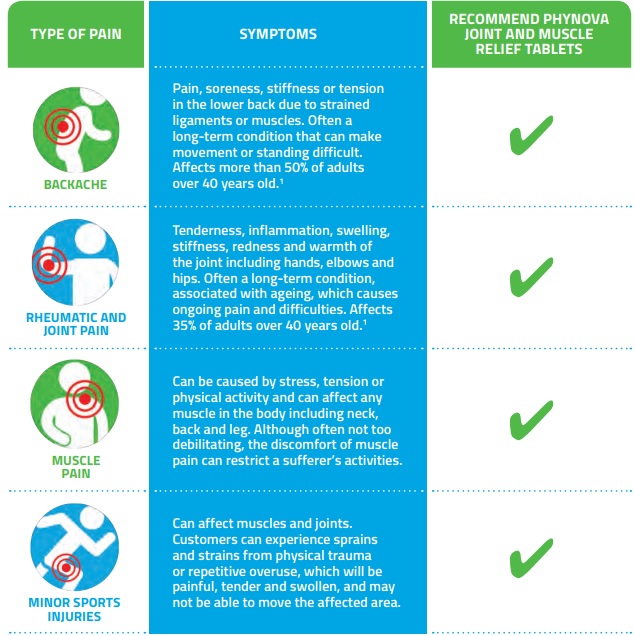
Patient Symptoms and Product Suitability
Pharmacy team training
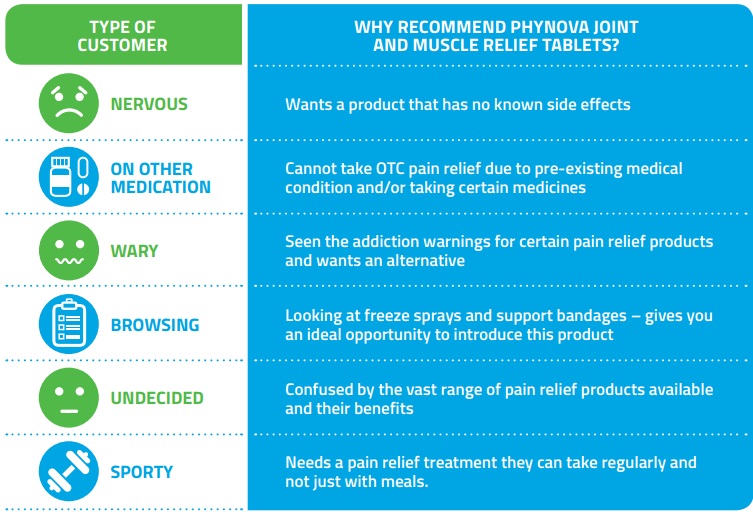
Understanding the Customer's Needs
Essential Information
Prescribing Information
Name of product: Phynova Joint and Muscle Pain Relief Tablets
Active ingredient: Dry extract of the aerial parts of Sigesbeckia orientalis L. subsp. pubescens (Makino) H Koyama. (equivalent to 4-5g of Sigesbeckia orientalis L. subsp. pubescens (Makino) H Koyama).
Presentation: Each pale yellow film coated tablet contains 500mg of extract from Sigesbeckia orientalis L. subsp. pubescens (Makino) H Koyama. aerial parts
Indication: A traditional herbal medicinal product used for the relief of backache, minor sports injuries, rheumatic or muscular pains and general aches and pains in the muscle and joints, based on traditional use only.
Legal category and product licence number: GSL, THR 41783/0001
Dosage and usage: Adults and elderly: Take one tablet, twice daily (one in the morning and one at night). Tablets should be swallowed whole with a little water or other liquid.
Side-effects: None reported
Contraindications: Hypersensitivity to Sigesbeckia or any plants of the Asteraceae (Compositae) family
Precautions for use: Do not exceed stated dose. The use in children under the age of 18 is not recommended because data are not sufficient. The safety of this product during pregnancy and lactation has not been established. The use during pregnancy and lactation is not recommended. The product should not be used by women who could become pregnant unless contraception is used.
Pack size and cost: Pack of 60 tablets £19.99 (incl VAT)
Name and address of product license holder: Phynova Group Limited. 16 Fenlock Court, Blenheim Office Park, Long Hanborough, OX29 8LN
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk. Adverse events should also be reported to Phynova Group Limited.


